اشترك في نشرتنا الإخبارية وكن دائمًا أول من يسمع بما يحدث.
Solution leaching process classification
Sep 12, 2024Solution leaching is a hydrometallurgical process that selectively dissolves minerals by placing the ore in an active chemical reagent of the leaching solution. It is a method of leaching that selects the appropriate metal extraction method based on economic and environmental feasibility. It is mainly used to extract metals from aluminum, nickel, cobalt, manganese and zinc ores.
Different leaching agents can be used in the metallurgical leaching process, depending on the target metal and its original matrix. The leaching agent can be alkaline or acidic. Sulfates can be leached with water or sulfuric acid, while oxides must use sulfuric acid or sodium carbonate solvents. Sodium hydroxide is used for oxides, while ammonium hydroxide is used for natural ores, carbonates and sulfides. Precious metals are dissolved in cyanide solutions, while some chlorides are dissolved in sodium chloride solutions.
Solution leaching can significantly reduce processing costs, enhance the sustainability of mines, and extend the life of mines by reducing cut-off grades or releasing value in mine tailings.
Classification of leaching processes:
1、In situ leaching
The leach solution is pumped into the rock formation (such as sandstone) at various points, and the solution is then pumped back to the surface at other points. If the ore body is not porous enough, it can be broken up first using blasting. This method of mineral extraction has long been used to recover soluble salt deposits deep underground. These are called evaporite deposits. This method has been used in the production of uranium and copper.
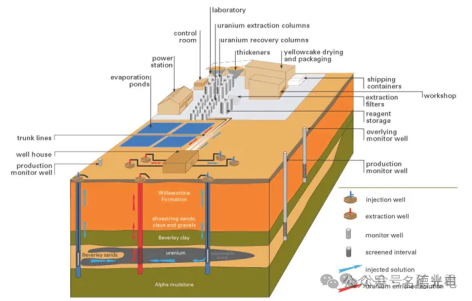
In situ leaching is suitable for fairly low-grade ores. Although the process reduces capital and some operating costs because there is no ore crushing, metal extraction tends to be low and the extraction rate is quite slow. ISL uranium mining was first tried experimentally in Wyoming in the early 1960s. The first commercial mine began operating in 1974. Today, almost all of Kazakhstan and Uzbekistan, as well as most of the uranium production in the United States, comes from ISL mining.
ISL can also be applied to other minerals, such as copper and gold. If the ore body contains a lot of calcium (such as limestone or gypsum, more than 2%), alkaline (carbonate) leaching must be used. Otherwise, acid (sulfate) leaching is generally better. In this case, the pH of the leaching solution is 2.5-3.0. Acid leaching provides a higher uranium recovery of 70-90% compared to 60-70% for alkaline leaching, and the operating cost is about half that of alkaline leaching.
2、Heap leaching
The ore is mined and piled up and treated with leachate from the top. The solution dissolves the ore and some gangue minerals and is collected at the bottom of the pile and processed for metal recovery.
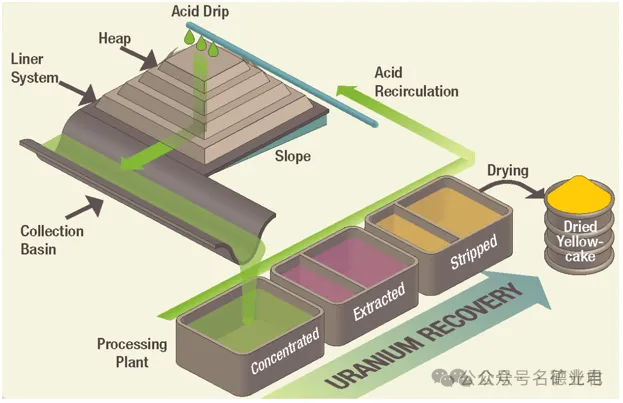
Heap leaching can use mined ore without further grinding, but usually first crushes it to a certain specified size range. Today, heap leaching is commonly used for gold, copper, uranium, nickel, zinc, etc.
Heap leaching uses a carefully constructed base or pad with a slope of about 1%. A heavy plastic liner is placed on the base and the ore is piled on top. The slope flows along the edge of the pile to a collection trough to collect the mother leach solution. The recycled leach solution flows from the top of the pile using large sprinklers and drip irrigation. A liquid pond is built downstream of the collection ditch for storage and buffering. The mother leach solution is extracted for metal recovery and the extracted barren solution is returned to the leaching process, with supplementary reagents added as needed. Heap leaching has an extraction efficiency of about 80%.
3、Dump leaching
Dump leaching is a method of economically extracting some valuable minerals from waste materials. Dump leaching is suitable for waste ores whose grade is too low for the main beneficiation process used. The size of the dump leached ore may exceed 1 m. Dump leach sites are located on natural slopes and the leach solution flows to natural watersheds. The dumps may contain millions of tons of ore, cover areas of hundreds of hectares, and may be >100 meters deep.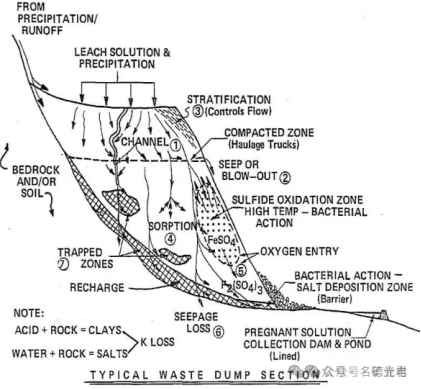
Dump leaching is a method of economically extracting some valuable minerals from waste materials. Dump leaching is suitable for waste ores whose grade is too low for the main beneficiation process used. The size of the dump leached ore may exceed 1 m. Dump leach sites are located on natural slopes and the leach solution flows to natural watersheds. The dumps may contain millions of tons of ore, cover areas of hundreds of hectares, and may be >100 meters deep.
4、Vat leaching
Vat leaching is the process where a liquid interacts with a metallic material in a vat or tank, which may be agitated to speed up the reaction kinetics. Crushing and grinding are often used to reduce the size of metal-bearing materials such as concentrates, ores, scraps, or slag prior to leaching. Agitators may also be added to the tank to keep solids suspended in the vat and increase solid-liquid interactions.Vat leaching and other methods minimize the environmental impacts associated with mineral mining by containing the ore within the tank or vat, reducing the footprint of the operation. Additionally, the use of alkaline cyanide solutions in reduction leaching can effectively recover metals while minimizing the release of harmful contaminants.
5、HPAL
Autoclave reactors are used for reactions at higher temperatures, which may increase the reaction rate. Likewise, autoclaving allows for the use of gaseous reagents in the system. Typically, ores and concentrates that are not suitable for atmospheric leaching, such as sulfides, are subjected to this aggressive form of leaching. High Pressure Acid Leaching (HPAL) is a process used to extract nickel and cobalt from laterite ore bodies. The HPAL process utilizes high temperatures (about 255 degrees Celsius), high pressures (about 50 bar or 725 psi), and sulfuric acid to separate nickel and cobalt from laterite ores. HPAL has been in use since it was first put into commercial production in Moa Bay, Cuba in 1961. The ore is mined and crushed to form a slurry, which is then preheated and pumped into an autoclave where the slurry and acid react. After leaving the high pressure and temperature environment of the autoclave, the slurry is washed and separated at atmospheric pressure to recover the metals from the liquid fraction.
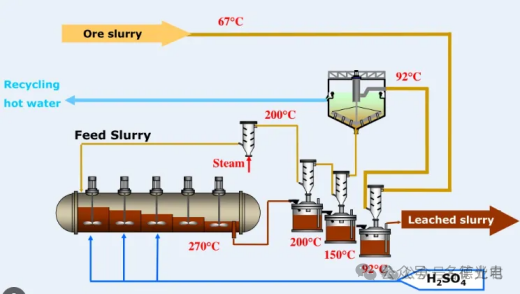
6、Tank leaching
The ore is placed in tanks and treated with leaching chemicals throughout the process. The precious metals are dissolved by the reagents, collected, and then purified. Tank leaching can be used for lean ores with low porosity. In tank leaching, the material is ground finer and the resulting slurry can flow under gravity or when pumped. The tank lining should be made of different materials depending on the treatment of different ores and the use of leaching solutions (various acids, alkalis, etc.), such as asphalt, acid-resistant concrete, lead sheets, epoxy resins, polyethylene plastics, and polymer materials. Storage tanks are usually equipped with agitators, baffles, and gas introduction equipment, designed to keep solids suspended in the slurry and achieve leaching.
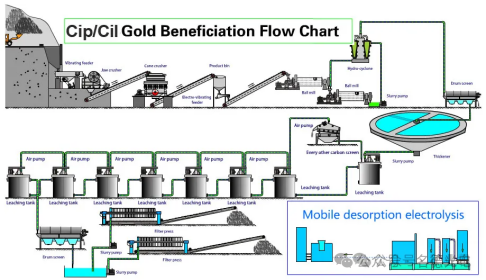
Metal Recycling:After the leaching operation, a mother liquor containing metals is obtained, which is generally treated by electrolytic deposition, metal ion transfer, chemical precipitation, solvent extraction combining electrolytic and chemical methods, and carbon adsorption combined with electrolytic treatment.
Electrolytic Deposition:Electrowinning gives a pure product and is a preferred method. Due to the high cost of electricity, solutions with a high metal content are generally processed. An insoluble anode and a cathode made of a strippable inert material or a thin sheet of deposited metal are inserted into a tank containing the leaching solution. When an electric current is passed through, the solution dissociates and the metal ions are deposited at the cathode. This common method is used for copper, zinc, nickel and cobalt.
Solvent Extraction: Solvent extraction is combined with electrowinning to concentrate dilute low-value metal solutions into small volumes and high metal content. Low-grade copper ores are processed in this manner by contacting a large volume of low-value copper leach solution with a small amount of a water-immiscible organic solvent in kerosene. The metal passes from the leachate into the extract solution, the two party separate, and the extract solution enters the extraction circuit.
اتصل بنا